Click here for a printable version.
Article 10 of the law on medicinal products (click here) allows companies to sponsor healthcare professionals for their participation in scientific events, whether this sponsorship is direct or indirect.
However, beMedTech (this is the Belgian federation of the medical technology industry) has decided internally to stop the sponsoring of individual participation in scientific events as of January 1, 2022 (click here for their press release of 24/11/2021 regarding this). As this is an internal deontological decision, it only applies to the member companies of beMedTech.
Important: this internal decision of beMedTech affects in no way art. 10 of the law on medicinal products. It is and remains permitted in Belgium to sponsor healthcare professionals for their participation in a scientific event in accordance with art. 10. If Mdeon receives a visa application, the Visa Office only applies the current legislation. Article 10 of the law on medicinal products provides for different ethical rules that are further elaborated in the Code of Ethics of Mdeon. The prior visa procedure was created as a guarantee for both companies and healthcare professionals that the sponsorship offered is ethical. Those who respect these rules will not be sanctioned by the authorities.
Doctors, pharmacists, nurses, vets, dentists, truss-makers, opticians, hospital directors, hospital equipment purchasers, chiropodists, midwives, laboratory directors, bio-medical operatives, wholesalers, physiotherapists, nutritionists, hospital technicians, clinical psychologists, etc.
I.e.: anyone in the healthcare sector who:
- distributes, purchases (or prepares for purchase), prescribes, recommends, delivers or administers medicinal products and/or
- distributes, purchases (or prepares for purchase), rents-out (or prepares for renting), recommends, uses or prescribes medical devices,
including institutions in which one or more of these activities take place.
In principle, it is not necessary to request a visa under these circumstances, unless the professional concerned exercises in his capacity as such in Belgium.
Companies which in Belgium
- manufacture, import, deliver and/or distribute medicinal products and/or
- have a marketing authorisation for a medicinal product and/or
- have a CE marking for a medical device and/or
- manufacture, import, deliver, distribute and/or rent-out medical devices,
- and invite or sponsor one or more healthcare professionals who practise in their capacity in Belgium to participate in an event of a scientific nature.
In other words, are concerned:
- both pharmaceutical companies and companies of the medical devices sector
- both Belgian companies and those from other countries
- both manufacturers and distributors, wholesalers or importers.
A visa is required once a healthcare professional practicing in Belgium is invited or sponsored by a pharmaceutical company or a company from the medical devices sector, whether or not the company is based in Belgium.
This is the legal wording coming from article 10 of the Law on medicinal products.
Two situations are concerned:
- The scientific programme of the event takes place over several consecutive calendar days. Ex.: it commences on day A and finishes on day B
- The scientific programme of the event takes place on one single day (or less) but requires an overnight stay as the event takes place abroad. Ex.: an event commences in Paris at 8.00am and finishes at 4.00pm. Participants must arrive the night before in order to be punctual for the start of the event. The event as a whole therefore takes place over several consecutive calendar days, even though the programme lasts for less than one day.
Yes, a visa is required as the programme alone already takes place over more than one consecutive calendar day.
If a company organises a scientific meeting lasting less than a day where only a dinner and/or a lunch is being offered and that is taking place abroad because of a congress at the same time to which all the invited healthcare professionals are participating, then no visa is required if:
- it lasts less than a day,
- it has nothing to do with the congress taking place at the same venue and its agenda and invitation were set and sent out in advance (this is prior to the congress taking place at the same time),
- it concerns an international public,
- only a meal is being offered.
Please note: in that case, Circular No. 622bis of the Federal Agency for Medicines and Health Products has to be respected.
If, on the other hand, it concerns the sponsoring of only meals during a congress, see FAQ 8.4.
A scientific programme is considered to be detailed when it contains per day:
- a detailed timetable (not only from 9.00am to 12 noon and from 1.00pm to 5.00pm but per units of 20 or 30 minutes, for example), also mentioning the date of the meeting.
- and a detailed content (not only the nature of the sessions but also the title of each scientific topic and, if possible, the names of the speakers). The content must be sufficiently clear so that the Visa Bureau understands what it is about. If too much jargon or uncommon abbreviations are used, this should be clarified to enable the Visa Bureau to properly exercise its control powers.
Please note that only programs of a purely scientific nature in the context of the medical and pharmaceutical science are allowed (see FAQ 5.1).
A preliminary programme may suffice with the proviso that it is detailed for each day in terms of content and timetable.
The company may submit a request for a visa as long as the following is included at the time of the submission:
- the provisional programme as available at the moment of submission of the visa,
- the detailed programme of the previous edition,
- and a confirmation by the organiser (by email) or by the company of the date at which the detailed programme will be available. This date must be no later than three months before the event; the company will otherwise have to await the publication of the detailed programme before applying for a visa.
After having obtained a visa on this basis, it is incumbent on the company to verify that the detailed programme, once available, does not differ in any way from the previous application. If this were not to be the case, a new request for a visa would have to be submitted as a result of the substantial modification to the programme.
However, the above-mentioned does not apply:
- to events taking place for the first time
- to events organised by a company
- to visa requests concerning sponsorship of scientific organisers (V2).
In these cases the request for a visa should only be made once the detailed programme (provisional or definitive) is ready.
As far as meetings for investigators are concerned, it is important to briefly explain in the visa submission (or in an appendix) what the clinical trials consists of and its aims. If uncommon abbreviations are used in the summary of the programme, they must be explained. Some meetings for investigators are in fact so full of abbreviations that the Visa Bureau is not in a position to verify if the event is a purely scientific one.
If the scientific event is organised by a company (or by a third party on behalf of a company), no social or cultural activity may be organised – even if healthcare professionals participating are paying for any such activity.
If the event is organised by healthcare professionals or other associations independent of companies, it is allowed to organise a social or cultural event, but only on the condition that
- it is not part of the scientific program (e.g. not during the day, but in the evening after the scientific sessions ended), and
- it is financed from other funds than those issuing from medical devices or pharmaceutical companies.
A purely virtual scientific event is subject to a visa requirement if it takes place over several consecutive calendar days and if its registration fee is paid (cumulative conditions). In this case, a supporting document of the registration fee (price + what is included) must always be attached: for more info see FAQ 9.4.
In the case of purely virtual participation, the hospitality offered is limited to the registration fee (meals or other hospitality may under no circumstances be offered).
For purely virtual scientific meetings, there is no minimum number of hours of scientific activities. For example, a virtual scientific program of 4 hours may be spread over 2 hours on day X (from 6 pm to 8 pm) and another 2 hours on day X+1 (from 8 am to 10 am).
Scientific modules that are available purely virtually at any time and therefore not at a specific time (e.g. e-learnings) are not subject to visa requirements. Indeed, sponsoring the registration fee to access such modules virtually is considered a gift (premium/benefit) and must therefore respect the conditions of art. 10 of the Medicines Act and art. 2 of the Code. Click here for more info.
For all types of events of a purely scientific nature: international congresses, meetings of investigators, expert meetings, life surgery meetings, local symposiums, hospital staff meetings, practical courses, virtual meetings, etc.
Certain topics, presentations or workshops can perhaps be interesting for the participating professionals, but are not in the context of the medical or pharmaceutical science. See for example presentations and workshops on financial, tax or social aspects linked to the exercise of a practice or “train the trainer” sessions. If such activities are planned, then also purely scientific activities need to be scheduled occupying the greater part of each day of the event.
In order to present an exclusively scientific nature, the activities of a scientific nature must always occupy the greater part of each day of the event, from the arrival until the moment of departure. One day must include at least six hours of scientific activities (excluding breaks), during normal office hours (and therefore not for example 7 am – 1 pm). For the first day and the last day, three hours may be sufficient to allow arrivals and departures.
Factory visits are not allowed, except in the following cases:
- Product or procedure training of medical devices that can only take place in the factory because of the relevant expertise or infrastructure in place;
- In the event of a major scientific added value for the participating professionals, which must be substantiated and justified. The motivation must prove, among other things, why the scientific event cannot take place in Belgium. Moreover, if the factory visit is to be preceded by presentations on theoretical aspects, the latter must represent a maximum of a third of the programme, if not the transfer abroad is not justified for the entire duration of the meeting.
Yes, as this is another form of sponsorship (that of participants and not of an organiser) which must be the object of a specific visa request via form « V1 – Sponsoring of Participants to a Scientific Event »
Yes, if the event is organised by an association (or on behalf of an association) managed by a majority of healthcare professionals practising in Belgium or if the majority of the participants are healthcare professionals practicing in Belgium.
.
A budget must consist of at least two columns: credit and debit. The credit column includes registration fees for the event (estimate), industrial financial sponsorship amounts, any subsidies, any pre-existing funding, etc. The debit column lists expenses linked to the event in detail. For a full example of what is required click here.
If the only source of income is coming from companies and the entire amount will be used by the organisers to cover all costs, the cost of the offered hospitality (lunch, dinner, lodging, etc.) must respect the maximum amounts mentioned in FAQ 8.1 and no social or cultural activity can be foreseen.
The budget does not necessarily have to balance. The organiser declares in any case in the visa submission that any eventual financial profit will be used according to article 10 of the law of 25 March 1964 on medicinal products. In addition, by submitting the visa application, the organiser commits himself to send the amount and allocation of the result of the closed accounts by email to the Mdeon Secretariat within three months following the end of the scientific meeting.
Sponsoring of events by the pharmaceutical and medtech sector can only happen if the sponsorship money is used exclusively to sponsor the scientific program.
In order to be able to demonstrate that the sponsorship money was exclusively used to finance the scientific part of the programme, organizers must be able to prove each item of expenditure separately on the basis of a quote, cost, invoices, receipts, origin of the money, sponsorship, profits, … If it is not possible to demonstrate this unambiguously in this way, sponsorship is completely excluded if non-scientific elements are also present.
These are scientific events organized by a third party (not a pharmaceutical or medical device company) whose program is exclusively scientific.
In that case, the organization of and participation in the scientific event is its main purpose.
The organization of the scientific part of these conferences may be sponsored. This includes speaker fees, room rental, logistics, staff rental, catering, invitations, etc.
As the sponsoring company, you must ensure that you have an appropriate agreement with the organizer stating exactly what the sponsorship money will be used for.
Please note that the presence of booths is tolerated but the sponsorship itself must relate to the organization of the scientific part of the congress. Moreover, booths may only be present in a separate room and therefore not in the congress halls.
2) Mixed events
These are events organized by a third party (not a pharmaceutical or medical device company) whose program is not exclusively scientific. This is the case, for example, when the publicity stands are not ancillary to the scientific part.
In such events, science is not the main reason for the event.
Such events may not be sponsored at all, even if the participants themselves would pay for the non-scientific part.
Pharmaceutical and medical device companies may not have a booth because this is a sponsorship of a benefit in violation of article 10 of the Medicines Act dated March 25, 1964.
3) Pure promotional events
The aim of these events is not to pass on scientific information, but purely promotional. Examples include trade fairs and exhibitions.
At such events, companies may attend with a booth if the following conditions are respected:
- There can be no scientific program (otherwise it is considered a mixed event, see point 2);
- No benefit (such as a workshop or non-scientific program) or gift may be given to participating healthcare professionals, nor may hospitality be provided at the booths;
- Booths are neutral and sober;
- No social activities may be offered (no party, reception, etc.) nor organized.
Approval would be given in such a case but healthcare professionals must then finance the additional dinner and overnight stay themselves as it would be possible to arrive in Paris on the same morning (leaving Brussels via Thalys, for example at 7.30am or 8.15am) and arrive in time for the start of the event (unless a particular reason for not so doing was declared in the visa submission). The same reasoning would apply for other destinations, such as Amsterdam, London, etc.
Arrival and leaving dates must coincide as closely as possible with the opening and closing times of the scientific event. Choosing a direct flight which would mean that the healthcare professional would come back later than if he or she had taken an indirect flight is, therefore, not acceptable unless the indirect flight does not present a reasonable alternative (for example where the indirect flight would arrive at destination in the middle of the night).
This would be acceptable but the healthcare professional would then have to finance the additional cost of the overnight stay and the evening meal him/herself -save for reasonable justification and motivation- as it would have been reasonably possible to return home before midnight.
- Meals may only be offered immediately before, during or immediately after a scientific activity (i.e., not along the way, at an airport or train station).Indeed, in accordance with Article 5.2 of Mdeon’s Code of Ethics, the hospitality offered must be strictly limited to the official duration of scientific activities. This means that a meal can only be offered during scientific activities, if it immediately precedes the start of scientific activities or immediately follows the end of scientific activities. Examples:
- It is permitted to offer a meal after a meeting abroad if it is impossible to return to Belgium that day and if the dinner coincides with the end of the scientific program (if the program ends before 5.30 PM, dinner cannot be offered).
- It is permitted to offer a lunch before the start of the scientific event, only if the scientific event starts before 2.30PM (if the program starts after 2.30 PM, lunch cannot be offered).
- There is a maximum of 45 euros for a lunch (drinks included), 90 euros for a dinner (drinks included) and 23 euros for a coffee break.
- However, a maximum of 135 euros per day with at least 6h of scientific program must be taken into account. If a day does not include 6 full hours of scientific program, a total maximum of 23 € per full hour of scientific activity may be spent on meals. The amount of 23€/full hour* of science is all inclusive (drinks, coffee breaks, VAT, room rental, etc.).
- Offering dinner on the eve of a scientific event may only be offered if there is a scientific program that eve.
* Calculation of the duration of the scientific program
- Maximum 23€ per full hour of scientific program may be offered for meals, to be divided proportionally over the entire time spent on pure science. A scientific program lasting 2h30 will thus entitle you to a meal of maximum 57,5€ (23€ x 2,5).
- Note: when calculating the duration of the scientific program, only the actual scientific program is taken into account. As a result, lunch breaks, coffee breaks, reception of participants, etc. without demonstrated scientific content may not be included in this calculation.
- The maximum amount of 45€ for a lunch, 90€ for a dinner and 23€ for a coffee break must be respected at all times.
- Amounts may not be transferred: e.g., if there is a scientific program of at least 6 hours but less than 135€ is offered in meals, the remaining amount may not be transferred to the day after or before. The amounts are calculated day by day based on the scientific program of that day itself.
EXCEPTIONS FOR CONSULTANTS
A participant is a healthcare professional who participates in a scientific meeting to learn.
A consultant is a healthcare professional who, in the context of a scientific event, performs scientific services on behalf of the event organizer or on behalf of a pharmaceutical/medical device company. Usually (but not always) this person receives an honorarium for this. Examples: speaker, researcher, expert meeting, etc. Is not a consultant: poster presentation, reporting afterwards, etc.
The following exceptions apply to consultants:
- Meals that do not directly follow the scientific program (or meals offered on the eve) and/or that are more expensive may be offered provided they are justified and described (amount to be specified) in a contract. However, the price of the dinner offered must always be reasonable/legitimate and comply with the authorized amounts as described above, unless there is a reason justified by circumstances to offer a more expensive dinner.
- A justifiable reason is, for example, a dinner with foreign consultants to avoid that only the Belgian consultants have to get another (cheaper) meal or are completely excluded from a meal, thus not being able to eat together with their peers and therefore not being able to talk about science. This reason must be explained in the visa application.
- Another justified reason is a consultant who is going to give a training of several hours in several hospitals (“speaker tour”). In that case, this consultant will also be able to get meals at times that are not directly related to the training insofar as this is contractually provided for (e.g. giving training every evening will henceforth also entitle him to receive a lunch).
- This contract will be attached to the visa application, which will also include the reference to the relevant article of the contract.
- The rules for consultants apply only to meals that can be directly linked to services performed in their capacity as consultants. If a consultant presents a topic at a conference but then further participates in his capacity as a participant, a difference should be made in hospitality offered.
- The capacity of consultant must be evidenced by supporting documents.
There is an exception for countries for which, according to the Ministerial Decree of January 10, 2023 (see FAQ 8.1 to consult this decree), the limit exceeds the maximum accommodation fee of 250 €. For these countries (and only for these), exceptions can be granted under the following cumulative conditions:
- At least 5 hotel offers are attached to the visa application showing that it is not possible to comply with the €250 price, and the cheapest offer is chosen;
- The hotels must be located within a reasonable distance (max. 10 km) from the place of the scientific event;
- The chosen hotel meets the reasonableness criteria as described in the Mdeon Code (article 5); for example, not known for its luxurious reputation.
Click here to consult this Ministerial Decree (p. 44-53).
Please note that the members of the Visa Office will verify that there are no reasonable cheaper options.
No, because the sponsoring must primarily be used for the sponsoring of a scientific event, which is not the case if only meals are offered. At least also something else that is necessary in order to take part in the scientific event must be sponsored (overnight stay, transportation, registration); if then additional meals are served, this remains accessory to the main objective.
However there is one exception: a lunch or dinner during a satellite symposium organised by the company and referred to on the official scientific program of the conference organiser.
If, on the other hand, it concerns meals offered during a scientific meeting that takes place over less than a day and that is organised on the occasion of but has nothing to do with a congress, see FAQ 3.3.
Only activities of a scientific nature may be sponsored. Consequently, if registration fees of a scientific event organised by healthcare professionals include a social activity (meals are not considered to be as such, contrary to a sightseeing tour or attendance at a show, etc.), there are two options:
- the company does not sponsor the registration fee,
- the company does sponsor the registration fee, minus the cost of these activities. Item B.4 of the visa application form allows this. If the organiser of the event has not stated the cost of the social activity, a reasonable estimate of the cost must be made.
When an event is organised by a company, hospitality costs must be indicated separately in the ad hoc section of item B.4 on the visa application form (lunch, dinner, coffee break, transport and overnight stays). The outstanding balance of the registration fee should appear in the section ‘registration’ (‘inscription’). In other words, the company must not simply state an ‘all-inclusive’ global sum per participant.
When an event is organised by a third party and the registration fee also includes hospitality, attention must be made to avoid any doubling-up. ie : the offering of the cost of a meal when this already appears in the registration fee (e.g. lunch symposium), which is not admissible even though the company would not sponsor the registration fee.
Yes, this is mandatory when the participation to a scientific event organised by healthcare professionals is sponsored, regardless of whether or not the registration is offered and regardless of whether or not there is a registration fee. Indeed, the visa application form asks to attach this justificatif even if the registration is not sponsored.
A justificatif must be added of 1) the price and 2) of what is included in the registration fee (lunch, dinner, social activity, etc.) and of its amount. If there is no registration fee, a justificatif of what the organiser offers as hospitality must be added.
The document to be attached has to be:
- either a print screen from the conference website (no email nor invoice of the organiser), that clearly mentions what is included in the registration fee (careful: make sure that the print screen is large enough to show the source of it)
- or, in the absence of information on the conference website, the congress organiser must complete the following model of sworn statement: model of sworn statement – registration. After completing the statement, the organiser must affix a handwritten signature and his stamp (in case of absence of a stamp, it must be printed on the stationery of the organisation), then convert the document to PDF and send it to the company to be attached to the visa application.
Example of what is included in the registration’s fee:
Registration fee includes:
- Access to the scientific sessions
- Congress material
- Lunches on day xyz
- Diner on day xyz
- Social activity (cost/pp is … EUR)
- Welcome reception
- Etc.
Please note that if it concerns a scientific event organised by a pharmaceutical or medical device company (even if it is not the submitting company itself), no justificatif should be added. Although, in that case, every offered hospitality must be detailed (€) in section B4 of the visa application (e.g. price of the offered lunch, dinner, coffee break, overnight stay, etc.), even if it is not offered by the submitting company itself (but, e.g., by another entity in the same group).
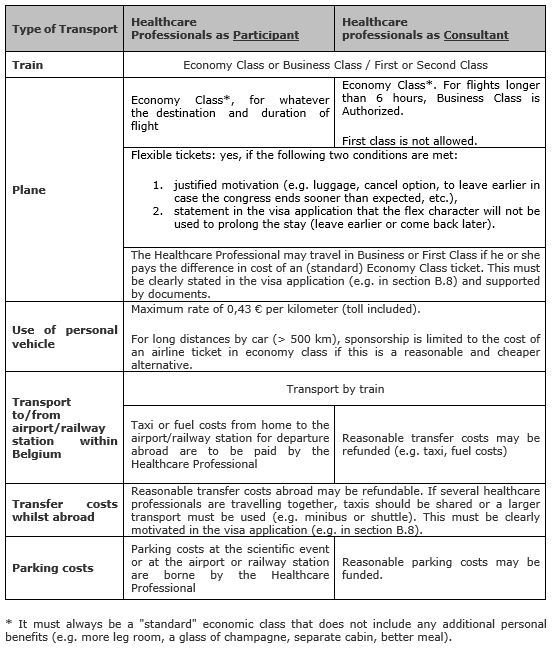
Train travel can be in either economy class or business class (second or first class).
Travel by plane happens basically in standard economy class. Travelling by plane in business class is only authorized if (cumulative conditions):
- the invited/sponsored healthcare professional participates in the scientific event as a consultant,
- and the outward and / or return flight is longer than a) six consecutive hours, b) or six non-consecutive hours because of a stopover, however, excluding the waiting time at the stopover in the airport (in other words, the consultant is at least 6 hours in the air).
Thus, regarding transport, a difference in terms of financing is made between participating healthcare professionals and those with the status of consultant. Here follows a definition of these categories:
- Participants: healthcare professionals taking part in a scientific event in a learning situation
- Consultants: healthcare professionals who are providing scientific services within the context of a scientific event on behalf of the organiser of the event or on behalf of a pharmaceutical or medical devices company. Examples: speakers, investigators, expert meeting, etc. The status of the consultant must be demonstrated in the dossier (e.g. name in program, defining mission in visa application).
See below for a summary:
10.1.b. May I offer flexible airline tickets?
Yes, provided two cumulative conditions are met:
- A motivated justification (e.g. flex character is needed to check in luggage, to cancel and recover costs, to shorten stay if the congress stops earlier, because there are no other options, because it is included by default in the chosen class, …), and
- A written statement that the flexibility will not be used to extend the stay (leaving earlier or returning later).
Both conditions (motivation and statement) must be mentioned in the visa application itself (e.g. in section B.5 and/or B.8). See here for an example: “We have opted for flexible tickets in order to be able to recover our costs in case of cancellation, but we declare that the flexible character will not be used to extend the stay“.
10.1.c. I’d like to reimburse taxi expenses: what to do?
Taxi costs should always be a realistic estimate of the actual price. When taxi costs are included in section B.4 of the visa application, an additional explanation must be provided in each case. This info could be, for example, the following:
- In case of multiple participants/consultants: will the taxi be shared?
- Distances to be covered?
- Regular taxi? Private driver ? Limo ?
- Add quote from an agency.
- Add simulation (see e.g. https://www.taxifarefinder.com/).
The healthcare professional must finance him or herself the difference in price with a (standard) Economy Class flight. This must be clearly stated in the visa application (e.g. in section B.8) and supported by documents.
If a flight ticket is sponsored, there has to be a justificatif of 1) the cost, 2) the class and 3) the date and hours of the chosen flight attached to the visa application. It has to be:
- Either a print screen from the reservation made online. On this document must clearly appear both the cost of the plane ticket and the class (e.g. not just class “E” or “Y”) as well as the date/hour (and if possible the flexible nature of the ticket or not). These information cannot be proven through an email of invoice of a travel agency. We do not expect of course a copy of a definitive reservation since the tickets are booked after reception of the Mdeon visa.
- Or a sworn statement completed by the travel agency the company works with, using the following model: model of sworn statement – flight. After completing the statement, the travel agency must affix a handwritten signature and its stamp (in case of absence of a stamp, it must be printed on the stationery of the agency), then convert the document to PDF and send it to the company by email to be attached to the visa application. Note that this sworn statement only applies to plane tickets booked through a travel agency. If a ticket is booked by the company itself, a print screen must be used.
Be careful:
- The cost of the flight mentioned in the visa application (section B4) must be exactly the same as stated on the justificatif and if appropriate, converted into euros.
- A sponsored flight should always leave from Belgium and return to Belgium. An exception may be allowed if the healthcare professional is elsewhere or should be elsewhere for professional reasons (e.g. participation in another conference), which must be justified and documented in the visa application. However, if it is for personal reasons, the flight can only be funded if this ticket is as a result not more expensive (unless the sponsored healthcare practitioner pays the difference in price himself).
- When working with a print screen, all the above mentioned data must figure clearly in one document. This document cannot be combined with another document.
The following may be financed: transport, meals and/or lodging that are strictly limited to the official duration of the meeting and also the cost of local transfers between the airport or railway station and the hotel/restaurant, if reasonable and motivated (e.g. taxi, distance, shared taxi, etc.).
The following may not be financed and must be paid for by the invited/sponsored healthcare professionals: drinks at the hotel bar, parking fees (exception for consultants), any passport or visa costs for entering a foreign country (unless limited to the period of the scientific event), use of a taxi or costs linked to use of a personal vehicle between home and airport or railway station for departure abroad (exception for consultants), snacks/lunch/dinner or refreshments at the airport or during the journey (with car or train), etc. Such costs are considered as being of a personal nature.
In all cases, hospitality costs must be reasonable and remain accessory to the scientific nature of the event.
A healthcare professional may obviously extend his or her stay for personal reasons but all additional costs linked to this prolongation are at the charge of the individual.
Yes, with the proviso 1) that transport costs are not higher than they would have been if the healthcare professional had not extended his or her stay (if this is the case, then the healthcare professional must pay the difference in price himself) and 2) that the duration of the extended stay remains accessory in relation to the duration of the scientific event.
The following table indicates when an extension of a stay is considered as to be accessory:
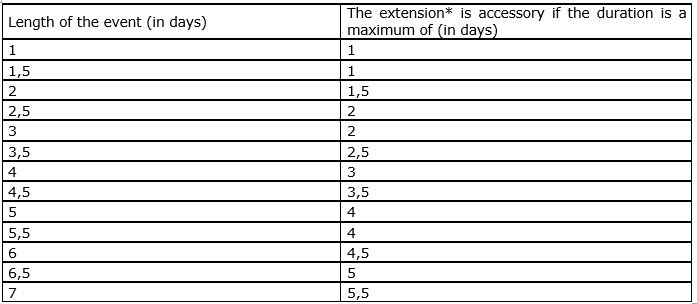
* By ‘extension’ it is understood all the days without scientific activity.
The company must limit its contribution as being towards transport costs linked directly to the scientific event. In concrete terms, the part paid by the company is limited to the fraction of the total cost obtained through multiplying the total travel costs by a fraction of which the numerator corresponds to the official duration of the scientific event in (half)days, and the denominator corresponds to the total duration of the stay, also counted in (half)days.
For example: a doctor participates in a scientific event in Rome for three days and then extends his stay by three days in order to visit the city – a total of 6 days. The company may only fund 3/6ths or half of this healthcare professional’s travel expenses, the other half being at the doctor’s expense.
The easiest way is to count backwards; eg: the scientific activities commence on Monday 2 May. If 15 working days are counted backwards from the first working day preceding, ie: from Friday 29th April and then not counting Monday 25th April (Easter Monday Bank holiday) the 15th working day preceding the event will be Friday 8th April. The request may be submitted on that day included, upto midnight (Belgian time).
One has to count backwards from the first official day of scientific activities, even if the participants do not participate on that day.
See also the tool “Calculation of the 15th working day” available on the Mdeon homepage, on the right in the gray frame.
- When the event gathers together a maximum of 15 participants and speakers of any different nationalities IN THE SAME PLACE. It should be noted that this figure represents the TOTAL number of persons concerned by the event (participants, speakers, company representatives – Belgian and foreign, all sessions included, etc..) The reason why the delay of 15 working days is not applicable in this case is because the event concerns a maximum of only 15 persons. In fact, the date of the event can be determined much more quickly than when an event concerns a larger number of participants.
- When the request for a visa is re-applied for following a substantial modification to the latter (after an initial granting of a visa).
- When a request for a visa is re-submitted following an initial refusal. It should be noted that this is only valid when the dossier was submitted within the time laid-down. It goes without saying that if the dossier was not submitted within the required time scale of a minimum of 15 working days, the applicant cannot use the reduced time delay of 6 working days.
- When the visa application concerns a healthcare professional taking part in the scientific meeting as a consultant, which must be demonstrated.
A reduced submission period of 2 working days applies in the case of emergency.
If you -for any reason- were unable to submit a visa application (V1 or V2) in time, you can still submit a visa application using the emergency procedure. This procedure means that you can submit a visa application (V1 or V2) using a shortened deadline of 2 working days prior to the first day of the scientific meeting, provided that the visa application is submitted not later than 12:00 (noon) on the second working day before the start of the meeting. The decision of the Visa Office will then be sent no later than the last working day before the start of the scientific meeting (midnight). A motivation why you are invoking the urgency procedure must not be provided.
For example: if the scientific meeting starts on Thursday December 17, the visa application must be submitted by Tuesday December 15 at noon at the latest. The decision of the Visa Office will then be sent to you on Wednesday December 16 by midnight at the latest.
Travel expenses: once a flight is sponsored (outward and / or return), the justificatif as referred to in FAQ 10.3 must always be attached.
Registration fee: once a company sponsors the participation to a scientific event organised by health professionals, the justificatif as referred to in FAQ 9.4 must always be attached, irrespective of whether or not the registration fee is sponsored and irrespective of whether or not a registration fee is requested.
If a company sponsors the participation of healthcare professionals in scientific events not directly, but through healthcare organisations (hospitals, scientific associations), it concerns an indirect sponsorship of scientific events. In this case, it will be the healthcare organisation that determines which professionals will benefit from the sponsorship.
If it concerns a sponsorship of participation to an event that takes place over several consecutive calendar days (including hospitality) (see FAQ 3), this indirect sponsorship is subjet to the visa obligation: see our brochure in this regard.
In this case, who submits the visa application: the company or the healthcare organisation?
You have the choice:
- the company can (continue to) submit the visa application itself, as the names of the invited healthcare professionals do not have to be mentioned in the visa application. The healthcare organisation will however have to provide the company with the necessary information to introduce the file.
- or the visa application is submitted jointly by both the healthcare organisation and the company: the healthcare organisation completes the visa application, encloses the necessary annexes and sends it to the company who checks it, pays for it and introduces it. To find out how to apply for a visa jointly, consult our operating instructions.
If you have received a visa number, you have to submit a new visa application if your project of sponsorship undergoes a substantial modification between the moment of submitting the visa application and the beginning of the scientific event (art. 22.1 of the Code of Ethics).
In this case a new visa application may be submitted at the latest the 6th or 2nd working day prior to the day the event starts (art. 17.4 and 17.5 of the Code of Ethics).
In general is considered as substantial every modification of which we can reasonably presume that the Visa office should take it into consideration in order to take a decision with full knowledge of the case.
Here are a few examples a substantial modifications, not limitative:
V1 – Sponsorship of participants
- the cost of the main transport (train/flight) increases by 50% or more or the transport class changes
- the number of sponsored participants
- increases by 3 or more persons (when you have already obtained a visa number for at least 4 persons) and the offered sponsorship remains the same
- at least doubles (when you’ve already obtained a visa number for 1 to 3 persons) and the offered sponsorship remains the same
- increases (independent of the number) and the offered sponsorship is different for the additional participant(s)
- the sponsorship is extended to costs other than those mentioned in the original visa application (addition of transport costs, costs for overnights,…)
- the hours of arrival or departure alter in an important way (see case by case) if the company sponsors the transport costs, even when the change in hours has no impact on the transport cost
- the scientific program alters
- the company sponsors one or more overnights extra
- the location of the scientific event alters
- the dates of the scientific event alter and this altering has an impact on the sponsoring
- in case of a grouped visa application concerning several identical events, an increase of the amount of times the event will take place
V2 – Sponsorship of the organiser
- substantial modification of the budget:
- additional sponsor(s)*
- extra costs in the budget (e.g. addition of a social activity)
- etc.
- alteration of the scientific program
- alteration of the location of the event
- the dates of the scientific event alter and this altering has an impact on the sponsoring.
* (!) The grouped visa number obtained by the organiser covers only the companies mentioned in the visa application and afterwards in the decision. If one or more sponsors present themselves later their sponsorship will have to be subject to a new visa application (substantial modification). This application can be submitted by the organiser as well as by the company itself. In this last situation, the file number of the organiser has to be mentioned in the visa application in order to allow the Visa Office to link the different applications.
Click here for the checklist.
L’Agence Fédérale des Médicaments et des Produits de Santé (A.F.M.P.S.) (The Federal Agency for Medicines and Health Products – F.A.M.H.P.). Mdeon is limited to ensuring an a priori oversight.
In this case contact should be made with the « Contact-Point » put in place by the authorities (02/524.83.58).
Both the sponsoring company and the healthcare professional receiving the sponsorship will be liable to legal proceedings (punishable by a prison sentence of between 1 month and 1 year and/or a fine of between 1.600 and 120.000 EUR).
In order that the two parties concerned (company and healthcare professional) can be sure that the applicable legal and code of ethic provisions have actually been respected (in view of the existence of penal sanctions which exist in case of non-respect of these provisions).
It is therefore essential that the visa number should appear:
- in correspondence exchanged between organisers and companies in the case of sponsorship of the organiser of an event
- in the correspondence exchanged between companies and healthcare professionals in the case of direct sponsorship of participants.
- Submit your visa applications using the Google Chrome browser
- Request your information technology expert to ensure that the e-mail addresses info@mdeon.be, visa@mdeon.be, and visum@mdeon.be are to be accepted and not placed in the spam bin.
- The keys Ctrl+F5 allow the programme to be refreshed
- Never use the key ‘&’ in the title of an appendix in an attachment to a visa request (nor in the ‘route’ leading to the document in your index).
- Certain headings appearing on the visa application form must be completed or the form cannot be sent. If one of these headings is not applicable in your case, the letters NA (not applicable) should be used in order that the form can be sent.
- Appendices attached to a visa request must not be greater than 4MB per annexe. If a scientific programme is any heavier, please send it in two parts in distinctly separate pdf documents.
- If you receive a message saying “this site is not secure??? it will be because your navigator does not possess a Network Solution certificate as used by Mdeon. In order to remedy the problem, you will need to accept our certificate via the link Network Solutions Add Trust External CA Root. Click on « open » then on « Install the certificate ».





